PETA India Outlines a New Deal to Revamp Laboratory Research
Animal experimentation has overwhelmingly failed to help humans suffering from disease. The US National Institutes of Health has stated that a “novel drug can take 10 to 15 years and more than $2 billion [USD] to develop, and about 95 percent of human studies fail”. Clearly, there is a major problem with the current paradigm for developing and testing drugs and getting them to market, and experiments on animals are one of the main contributing factors.
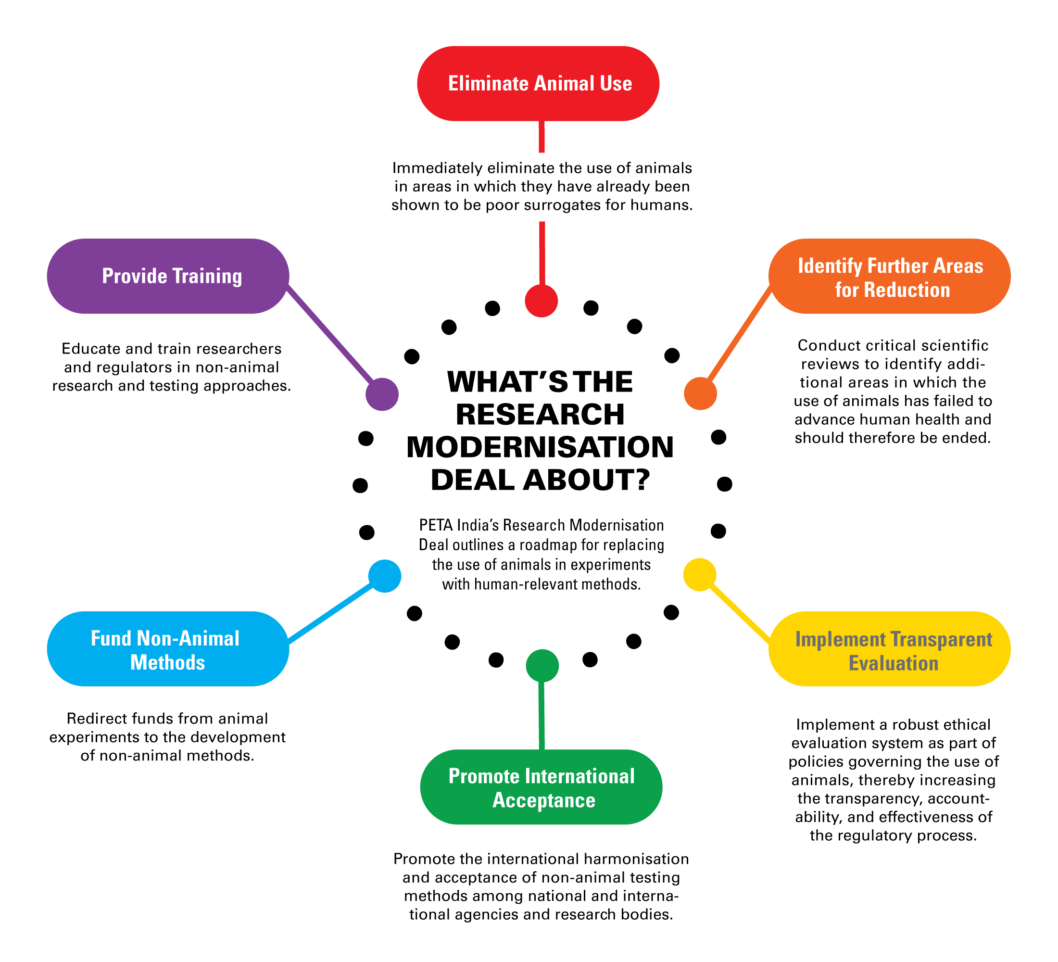
To address this issue, PETA India scientists have developed the groundbreaking Research Modernisation Deal: a road map for strategically optimising India’s investment in research to cure disease by ending funding for strategies that don’t work, including experiments and education using animals, and instead directing resources towards research that’s relevant to humans, such as accelerating the uptake of advanced technologies that outperform animal-based methods. PETA India is calling on the government to embrace this new plan to reform the broken medical research system.
The Current System Is Broken
The US National Institutes of Health confirms that 95% of all new drugs that test safe and effective in animal tests fail in human clinical trials, most because they don’t work or actually turn out to be dangerous.
The failure rates of new drugs developed using animals are even higher in specific disease research areas:

HIV vaccine: 100% failure rate

Strokes: 100% failure rate (based on 1000 new agents tested in animals in 100 clinical trials)

Alzheimer’s disease: 99.6% failure rate
Cancer: 96.6% failure rate
Furthermore, the majority of “highly promising” basic science discoveries are based on animal studies, but fewer than 10% of these enter clinical use within 20 years. This matters because the failure to reproduce preclinical research equates to more than Rs 2,11,405 crores per year being spent on misleading experimentation and unacceptable delays in delivering effective treatments.
We need a better way – and PETA India scientists have come up with it: the Research Modernisation Deal.
What's The Research Modernisation Deal About?
PETA India opposes all experiments on animals and campaigns for an immediate end to all animal use. Regulatory and scientific authorities must acknowledge the failure of animal studies and, as a first step, immediately end all animal use in disease research areas in which it is known to fail to produce results that help humans. To do otherwise is unethical, impedes good science, wastes resources, and harms animals.
What You Can Do
Now is the time for India to reaffirm its role as a world leader in biomedical research and regulatory testing by shifting away from failed, cruel and wasteful experiments on animals and embracing the change to human-relevant models that will bring desperately needed treatments, cures, and vaccines.
Please sign our petition to show your support of The Research Modernisation Deal and request that the prime minister work to establish a clear policy mandating an end to animal experimentation and provide a strategy and timeline for achieving this goal.